- Experiment II - Solutions & Dilutions. And biology use solutions and their dilutions in the lab and in the field, so knowing how to prefom a dilution is a very.
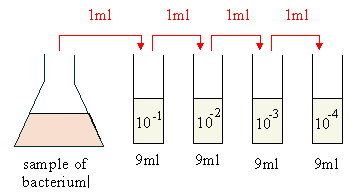
- A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. The easiest method is to make a series of 1 in 10 dilutions.
LAB 4: ENUMERATION OF MICROORGANISMS. State the formula for determining the number of CFUs per ml of sample when using the plate count technique. Perform a serial dilution of a bacterial sample according to instructions in the lab manual and plate out samples of each dilution using the spin-plate technique. Documents Similar To Lab Report. Serial Dilution. Discussion MICROBIOLOGY SIMPLE STAIN. Gas pressure process control lab report. Preparing and Diluting Solutions Lab. Using the molarity and dilutions equations. The relationship between the concentration of the solutions and their absorbance will be investigated. The accuracy of the solution preparation and dilution procedures will then be. Section of your Results for this lab and indicate that that is the amount of. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. The easiest method is to make a series of 1 in 10 dilutions.

Serial Dilution Lab Activity

Serial Dilution Lab
Introduction
The purpose of this lab is reach and be able to calculate the equivalence point when we use titration to neutralize a base with acid. The process of the lab was determining the volume of a solution needed to react with a given mass or volume of a sample is called titration. The equivalence point is when the same number of moles of acid and moles of base has been added. Phenolphthalein is used as an indicator because it will have a color change when the equivalence point has been reached. Procedure
See AP Chemistry. Chapter 4, Acid-Base Reactions, Titration Lab for procedure, or see worksheet. Data
Standardization of Base with Solid Acid |
PART I| Solid Acid| Base (NaOH)| Ratio: volume base/ mass acid| Final mass or volume| .70g| 38 ml| 38 ml / .70g |
Initial mass of volume| .70g| 0.0 ml| |
Mass or volume| .70g | 38 ml | |
Titration of HCL |
PART II| Acid (HCL)| Base (NaOH) | Ratio: volume base/ volume acid| Final volume| 10 ml| 4.8 ml| 4.8ml / 10ml|
Initial volume| 0.0 ml| 0.0 ml| |
Volume| 10 ml| 4.8 ml| |
Calculations Part I
Solid Acid- KHC4H4O6- 188g - .0037mol Base- NaOH .038 L 1 KHC4H4O6 + 1 NaOH > H2O + NaKC4H4O6
.0037mol KHC4H4O6 *1 mol NaOH/ 1 mol KHC4H4O6 = .0037 mol NaOH= MnaOH = .0037 mol/ .038 L = .0974 MnaOH
Calculations Part II
M a * V a = M b* V b
M a * .010 L = .9074 M * .0048 L
(.0974 M * .0048 L) / .010 L = .047 M a = .047 M of HCL
Conclusion
The Purpose of this lab was to find the equivalence point of a titration of an acid and base. We were able to find that the concentration of NaOH that was needed to neutralize our solid acid was .0974M and that a concentration of HCL .047M was needed to neutralize.0974 M NaOH. We were able to calculate this with the equation of M acid * V acid = M base * M base. The same lab was done by www.chemicalforums.com and found that they needed .077M of HL was needed to neutralize their NaOH. There is...